Northwestern scientists publish guiding principles for COVID-19 vaccine development
A group of Northwestern University scientists including Simpson Querrey Institute (SQI) members Michael Jewett and Samuel Stupp has created a set of guidelines to assist in developing a safe and effective vaccine for COVID-19. Published July 21 in ACS Central Science, the principles were gleaned from early research on COVID-19 and data from the 2003 SARS outbreak, which was caused by a virus (SARS-CoV) very similar to the one that causes COVID-19 (SARS-CoV-2).

“When the pandemic entered the U.S., we started thinking about how we could contribute with the tools that we have and felt a responsibility to do what we could to help,” said Ariel Thames, an MD-PhD candidate in Jewett’s lab and first author of the paper. “We tried to synthesize a framework for those serving on the front lines so that they could understand the immunology and let that inform vaccine design, patient care and treatment development.”
Kristy Wolniak, assistant professor of pathology at Feinberg School of Medicine, offered valuable insight on the immunology of the virus, and all four authors played a role in framing the key points in the paper.
Here are four important takeaways from the research:
1. A COVID-19 vaccine must strike a delicate balance.
In the case of COVID-19, studies have shown that a broad immune response may trigger inflammatory processes that damage the lungs, leading to acute lung injury. A successful vaccine will likely require eliciting specific antibodies — proteins that respond to and neutralize pathogens — and T cells without invoking a large-scale inflammatory response.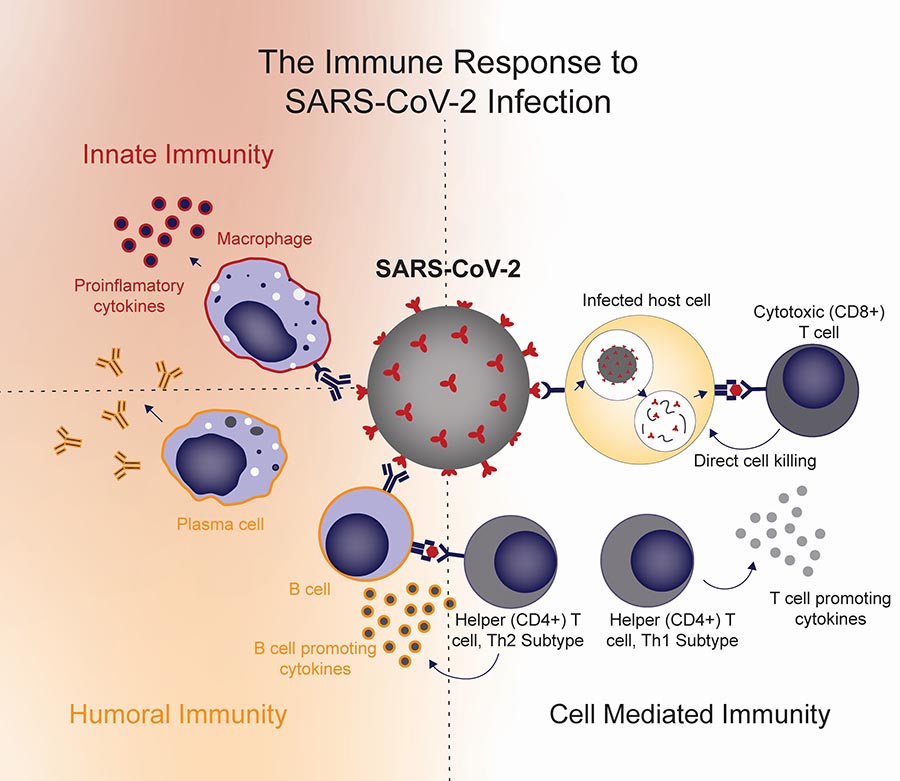
A strong T cell response has been associated with both clearing the virus and guarding against lung injury. T cells may also help the immune system “remember” the virus for longer periods of time than antibodies alone, so a vaccine’s ability to activate this response could be a key factor in long-term protection, the authors determined. Monitoring for a T cell response to a vaccine is much more challenging than monitoring for an antibody response, but it may critical in measuring true COVID-19 protection.
“To prevent lung injury, you want to elicit an effective immune response but nothing extra, so that you’re not activating a big cascade of immune processes that aren’t as helpful,” Thames said. “By primarily eliciting a T cell response and getting that antibody specificity correct, you should be able to effectively clear the virus and circumvent any of these harmful effects.”
That’s not to say the process is easy. Even with the playbook offered by the Northwestern scientists, a successful vaccine will have to hit the correct parts of the immune system at just the right strengths.
“It’s finding a needle in a haystack, and it’s doing that under immense time pressures that frankly have not been seen in our lifetimes,” said Jewett, Walter P. Murphy Professor of Chemical and Biological Engineering and director of the Center for Synthetic Biology at Northwestern. Jewett was also corresponding author on the paper. “Getting all of those bits right is what hopefully the framework of this article will provide, enabling scientists and perhaps even the non-expert to begin to understand how hard of a problem this is.”

2. Antigens and vaccine platforms provide opportunities to “tune” immune responses.
Strategically varying these two factors, Jewett said, offers scientists the best chance to find the right combination for a safe and effective vaccine. Antigens are the molecules — often proteins — that are detected as a foreign substance in the body and can trigger an immune response. Vaccine platforms can take many forms, each of which causes the body to react differently.
“You can have a viral vector vaccine, a nucleic acid vaccine or protein-based vaccines, and all of those will activate slightly different parts of the immune system,” Thames said. “They all overlap in some ways but some platforms will activate one branch of the immune system more strongly than the other.”
3. Clinical trialists should monitor for lung injury after vaccination.
Given the observation of immune-mediated lung injuries in patients and animal models with SARS-CoV and SARS-CoV-2, the authors believe these conditions must remain top of mind for clinical trialists and medical professionals.
“We hope this article can positively influence medical practice,” Jewett said. “It can inform the evaluation of current vaccine candidates and clinical trial development, not just for COVID-19 but for other vaccines.”
4. COVID-19 data are ever-evolving — and so are researchers’ efforts to fight it.
Jewett said the manuscript took a few months to prepare and required weekly updating due to the endless deluge of new data. The authors aren’t claiming to know how to elicit the optimal immune response in a COVID-19 vaccine, but they believe the basic principles they’ve identified — based on the best available information to date — can help the scientific community eventually reach that point.
“Having 10s of vaccine candidates already in human clinical trials, and many in the pipeline, in just months — it’s really been remarkable,” Jewett said. “As scientists, this is one of our chances to try to transform the way we exist on the planet in the wake of a pandemic.”
Jewett is inspired by the way scientists have responded to the COVID-19 outbreak. He need only look inside his lab for an example.
“What’s so awesome as a PhD advisor is when you have innovative and creative students that aren’t willing to sit on the sidelines,” Jewett said. “When campus was shut down, Ariel said, ‘Well, I can’t do my research, but let me see if I can synthesize and contribute meaningful information to the development of how we’re going to address COVID-19.’ ”
“I think that just really speaks to the caliber of a scientist that Ariel is and more broadly the scientists that we have at SQI and Northwestern.”
