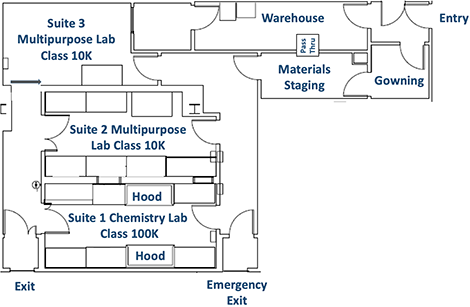
Pilot GMP Laboratory
The Peptide Synthesis Core manages the use of a Pilot Good Manufacturing Practices (Pilot GMP) Laboratory in the Simpson Querrey Biomedical Research Center. This laboratory simulates the controlled production conditions required for therapies to be used in clinical trials, and is intended for investigators who are interested in scaling up processes or testing projects that are promising candidates for clinical development.
Appropriate use of the laboratory will enable production of materials under GMP-like conditions that may then be useful for pre-clinical experiments or data collection. While this lab is not an official GMP-compliant facility, it is constructed to GMP standards and designed to simulate GMP environmental conditions.
Users may reserve the laboratory for approved projects. Fill out the online application form through the link below to request use of the space. Please contact Mark Karver at mark.karver@northwestern.edu if you have questions about the Pilot GMP Laboratory.

Suite 1: Chemistry Lab Class 100K